Software as a Medical Devices (SaMD) and mobile health (mHealth) applications have revolutionized the healthcare landscape in the areas of Remote Patient Monitoring (RPM) and Digital Therapeutics (DTx). These technological advancements offer a range of benefits, from improved patient engagement and real-time monitoring to personalized treatment plans and enhanced clinical outcomes. During the continuous design, development, and validation of our medical device Healthentia, we have conducted a comprehensive systematic literature review to assess the current status and efficacy of these digital health interventions and benchmark the results with our finding from the clinical evidence we have collected from various studies. The results are rather positive, demonstrating how SaMDs like Healthentia can support the management of chronic diseases, satisfying patient safety and performance requirements.
The current landscape of SaMD and mHealth apps is characterized by rapid innovation and widespread adoption, especially after the Covid pandemic, which might have worked as a catalyst for digital transformation in digital health. SaMD refers to software intended to be used for medical purposes that perform these purposes without being part of a hardware medical device. mHealth applications, on the other hand, encompass a wide range of digital tools designed to support health and wellness through mobile devices. mHealth applications are not necessarily medical devices and therefore, they often don’t comply with the regulatory framework. Both SaMD and mHealth apps have seen significant advancements, driven by the increasing need for accessible, efficient, and patient-centric healthcare solutions. Regulatory frameworks have also evolved to keep pace with these innovations, ensuring that these technologies meet rigorous safety and efficacy standards. While the potential of SaMD and mHealth apps are immense, there are several challenges that need to be addressed to fully realize their benefits, such as data privacy concerns, integration with existing healthcare systems, and user adoption barriers.
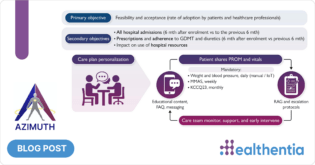
The review involved a scientific process of identifying, selecting, and analyzing relevant studies that evaluate the effectiveness of SaMD and mHealth apps in managing chronic diseases, using the PICO (Population, Intervention, Comparison, Outcomes and Study) framework. The selected studies provide a robust evidence base, highlighting the positive impact of these technologies on patient outcomes.
This post aims to provide a comprehensive overview of the current status of SaMD and mHealth apps, highlight promising results, and discuss the potential these technologies hold for improving health outcomes and provides only an overview of a peer reviewed paper that we expect to publish in the coming months.
The safety and performances of such solutions for chronic disease patients is thoroughly discussed in various meta-analyses that have been retrieved and analyzed and include 14 meta-analyses for diabetes Type II, cardiovascular diseases, COPD and cancer. Those meta-analyses allow to identify the main clinical performances that could be expected for Healthentia to define the acceptance criteria for the risks/benefits acceptability. In addition, clinical studies originating from the selected systematic reviews were analyzed to check which features were put in place in the eHealth solutions under evaluation, allowing a comparison with those of Healthentia, resulting in prospective controlled studies on several chronic diseases:
- Diabetes Type II: 24 studies, about 1.200 patients in the intervention group
- COPD: 1 study, 36 patients
- Cancer: 3 studies, about 150 patients
- Heart diseases: 7 studies, 560 patients
Summarizing the results from these studies we provide some data both biomarkers and other clinical endpoints that demonstrate the potential:
- HbA1c level: Decreased in several of the studies.
- Physical activity & performance: Significant improvements in exercise capacity.
- Body fat: Significant improvements.
- Sleep: Significant improvements in sleep time.
- Self-care/management: Self-reported improved the self-monitoring of patients’ blood glucose levels, diet, exercise, other self-management skills, and knowledge of the disease.
- Depression & anxiety: Self-reported improved effect on their psychological status.
- 6MWD: Significant improvement.
- (HR)QoL: Difference between the DQoL–Social/Vocational Concerns subscale scores was statistically significant.
- Hospital readmissions: The 30-day hospital readmission rate was much lower, compared with the national readmission rates.
- Adherence: Significant improved medication adherence.
The list of results above is indicative and is only provided to showcase the potential and is not to be considered as a scientific outcome itself. Scientific results will be presented and analyzed in the Systematic Literature Review paper in the coming months, which will also include evidence and findings from studies that used Healthentia. By sharing our findings and experiences, we hope to contribute to the ongoing dialogue on digital health innovations and their role in transforming chronic disease management. Furthermore, the insights gained from the literature review and our benchmarking analysis may offer valuable guidance for healthcare providers, patients, and policymakers as they navigate the evolving landscape of digital health.
Looking ahead, the future of SaMD and mHealth applications appears promising, with the potential to significantly enhance the quality of care for patients with chronic diseases.
Sofoklis Kyriazakos, CEO