Healthentia is now listed in mHealthBELGIUM, the Belgian platform for mobile applications that are CE-marked as a medical device. mHealthBELGIUM is an initiative of the Belgian Federal Government managed by beMedTech (sector federation for the industry of medical technologies) and Agoria (sector federation of technological industry) in close cooperation with three national authorities: FAMHP (Federal Agency for Medicines and Health Products), eHealth Platform (a federal government eHealth institution) and NIHDI (National Institute for Health and Disability Insurance).

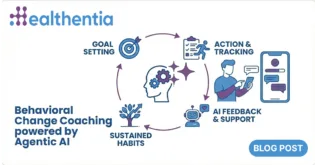
Healthentia is an innovative eClinical solution that goes beyond the typical ePRO/eCOA context, blending sensing information with traditional outcomes to discover lifestyle digital biomarkers for certain phenotypes in clinical studies; thus enabling smart eHealth services. Currently Healthentia is a Class I Medical Device; a Software intended for monitoring of non-vital parameters to support decision making during clinical trials. Further to the current certification, on our roadmap towards a Class IIa Medical Device, Healthentia R&D features include novel approaches towards DTx services that exploit the derived digital biomarkers to feed novel intervention.