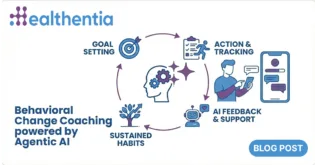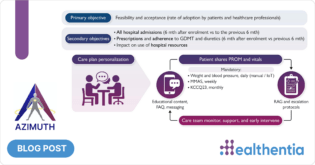
Undoubtably, AI was the keyword that dominated technology and new product reviews across all industries for 2023. This comes as no surprise, as AI was offered to the masses like never before – mainly through ChatGPT.
AI has been characterized over the past many decades from several periods of high activity and outputs, followed by “AI winters.” Today we obviously experience a high activity period with lots of outputs and 2023 has been very AI intense, because everyone with Internet access was able to validate its capabilities.
Life Sciences is among the industries with the highest impact of AI, but at the same time one of the most regulated ones. Large Language Models (LLMs) like GPT-4, LLaMA and Gemini are already used for various health and clinical applications like drug discovery, clinical protocol design, documentation, image analysis, task automation, virtual assistant and many more and the expectation is that these will grow much in the coming years. At the same time, regulators around the world are working hard to create the appropriate framework to control AI, in a way to promote transparency, prevent discrimination, protect privacy, enforce safety, and foster responsible innovation. Recently, the AI Task Force of the eClinical Forum and the European CRO Federation (EUCROF), published a white paper presenting the state of play and providing information about AI and ML in clinical research around the globe, with emphasis in US and Europe. Sofoklis Kyriazakos, leader of the AI joint Task Force, says “This white paper is an important milestone for the industry that sees the benefits of AI and puts efforts to understand the environment and how to transform the challenges into opportunities. This has been a very nice team work that underlines the importance of joining forces to make a step forward and adopt AI technologies.”
The year 2024 will start with the stakeholders of the industry being widely exposed to the capabilities of generative AI, most of them having invested resources to investigate or even to deploy such solutions and being more realistic on other matters with emphasis on the feasibility and compliance. We expect that 2024 will be the year of design, development, and compliance for the industry to create solid grounds for the adoption of AI. In Innovation Sprint we perform high impact research and development to identify AI features that can add value to Healthentia and follow a thorough process for implementation to become commercially available in minimum time.